THE MASTER OF SCIENCE IN CLINICAL RESEARCH PROGRAM: PREPARING CLINICAL RESEARCH PROFESSIONALS
By Gene Dillague, MD, MSCR, CCRA, Dean
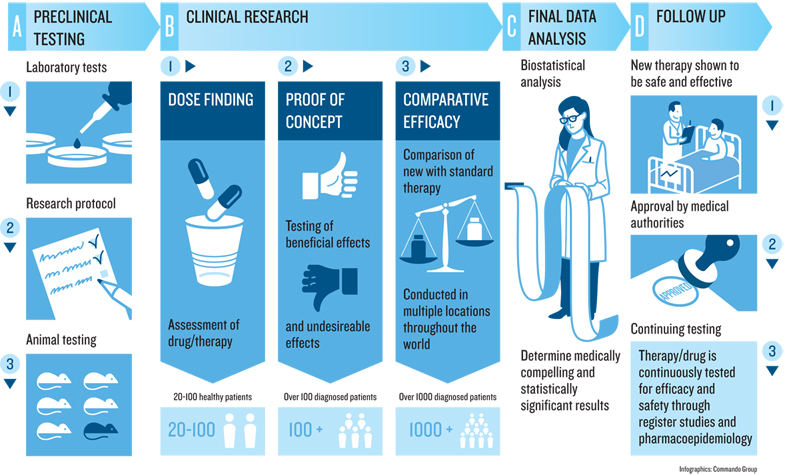
Drug discovery and drug development are essential to the creation of therapeutic solutions which all heath care professionals employ in the art of healing. These involve complex processes that begin with the discovery of the new drug molecule, its testing in cells and animals, and, finally, its trials in humans. Proving that the new drug molecule is both safe and effective for the indication requires extensive clinical trials in human subjects and patients. This endeavor is highly regulated by the government to ensure that all drugs approved are both safe and efficacious. While physician-investigators conduct the trials, clinical research professionals facilitate, oversee and manage them at the clinic or the institution.
The MSCR program prepares and equips future research professionals by training them in three critical areas: medical science, regulatory compliance and project management. The program trains our students in fields of anatomy, physiology, medical chemistry, pathology and pharmacology to ensure their ability to understand the clinical trial protocols and its nuances. It also equips them with the solid understanding of federal laws and regulations to ensure compliance. Finally, the program hones their skill in project management, site monitoring and oversight.
As competent clinical research professionals, our graduates work as study coordinators or project managers for clinical research sites; as site monitors, study managers or project leaders for pharmaceutical or medical device companies; and/or as regulatory and safety compliance officers for the government.