

ACRP-Southern California Chapter
-
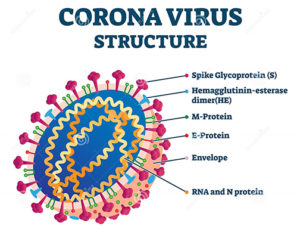
COVID-19: A Brief Overview
By: Balwantsinh Chauhan, MD, PhD Associate Professor, School of Pharmacy Coronaviruses are introduced as a group of related RNA viruses…
-

Repentance
Sin is an action that we perform on a daily basis. At times, we are unaware of committing these sins…
-

Teaching and Learning In A Social Distancing Environment
By: Jonathan Sheng, PhD Assistant Professor, Department of Pharmaceutical Sciences, School of Pharmacy The recent COVID-19 event has posed great challenges…
-
Understanding Pharmacogenetics of COVID-19: Role in Potential Magic Bullet Drug
By: Dr. Sukhwinder Lakhman Assistant Professor, Department of Pharmaceutical Sciences, School of Pharmacy Immune responses demonstrate a high level of…
-
How Coronavirus Affects Community Pharmacy Operations
By: Christine Han, Pharm.D Assistant Professor, School of Pharmacy Commitment to their community drove many pharmacists to go above and…
-
Surviving Covid19: Kidney Transplant Recipients Perspective
By: Young il Chang, PharmD. MS. Assistant Professor, Department of Clinical and Administration Sciences The post-transplant patients, as you know,…
-

It’s Hard For Me to Breathe
IT’S HARD FOR ME TO BREATHE by: Pastor Gregory Johnson It’s hard for me to breathe sometimes When people look…
-

Developing AUHS Pharmacy Students as Community Advocates
At the American University of Health Sciences (AUHS) School of Pharmacy, as an on-going effort for continuous engagement with legislators,…
-

Acts of Love – November ’19
In November, AUHS expresses gratitude to God through giving and serving others so that they can enjoy the Thanksgiving holiday.…
-

AUHS To Host ACRP Southern California Chapter Workshop Highlighting Morisky Medication Adherence Scale In Clinical Research
UPDATE 6/30/19: Click below to watch a recording of this event Participants will learn to conceptualize strong scientifically validated research…
-

University Update – January ’19
On Thursday, January 31st, 2019, faculty, staff and students gathered at the conference room on the second floor for the…
-

AUHS Honored to Host Ms. Harriet Glickman
Franklin Armstrong is a character in the long-running comic strip Peanuts, created by Charles M. Schulz. On Saturday, November 17,…