12th ISCTICO, WCP2023, Glasgow, Scotland
At the 12th ISTICO conference, organized by two AUHS faculty, which was a satellite meeting of the WCP2023 in Glasgow, Scotland, three AUHS faculty made presentations: Profs. Oksana Zayachkivska, John Schloss, and Sandor Szabo. Short abstracts and representative slides of the presentations are below.
12th ISCTICO Glasgow Program.final.rev.2.
Cysteamine & its derivatives: From structure-activity & secretory/motility studies to the critical role of iron in the pathogenesis of duodenal ulceration.
Sandor Szabo, MD, PhD, MPH
To understand molecular pathogenesis, i.e., the sequence of events before we can see signs & symptoms diseases, we need appropriate animal models of human diseases. Namely, studying a disease in patients may reveal why a disease is active, & if it will respond to certain therapeutic interventions. Thus, only in animal models we may gain insights into molecular & cellular mechanisms of development of diseases.
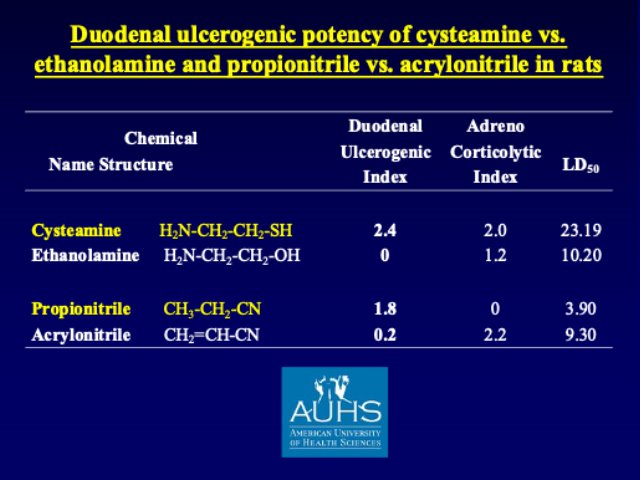
 Duodenal ulcers (DU) are the most frequent form of ‘peptic’ ulcers, yet until about 40 years ago there were no easily reproducible animal models of DU. During my PhD-related experimental work at the University of Montreal I recognized that simple chemicals, like propionitrile & cysteamine produce rapidly developing perforated DU in rats & mice. Time-sequence studies with these chemicals allowed us to detect early biochemical alterations not only in the gastric & duodenal mucosa, but also in certain brain areas & adrenal glands. Our structure-activity studies with derivatives of cysteamine & propionitrile revealed that the duodenal ulcerogenic potency of these chemicals depends on having nucleophilic radicals (e.g., -SH, -CN) on 2-carbon backbones. Thus, replacing -SH on the 2-carbon cysteamine with -OH (i.e., ethanolamine H2N-CH2-CH2-OH) results in total loss of DU potency.
Duodenal ulcers (DU) are the most frequent form of ‘peptic’ ulcers, yet until about 40 years ago there were no easily reproducible animal models of DU. During my PhD-related experimental work at the University of Montreal I recognized that simple chemicals, like propionitrile & cysteamine produce rapidly developing perforated DU in rats & mice. Time-sequence studies with these chemicals allowed us to detect early biochemical alterations not only in the gastric & duodenal mucosa, but also in certain brain areas & adrenal glands. Our structure-activity studies with derivatives of cysteamine & propionitrile revealed that the duodenal ulcerogenic potency of these chemicals depends on having nucleophilic radicals (e.g., -SH, -CN) on 2-carbon backbones. Thus, replacing -SH on the 2-carbon cysteamine with -OH (i.e., ethanolamine H2N-CH2-CH2-OH) results in total loss of DU potency.
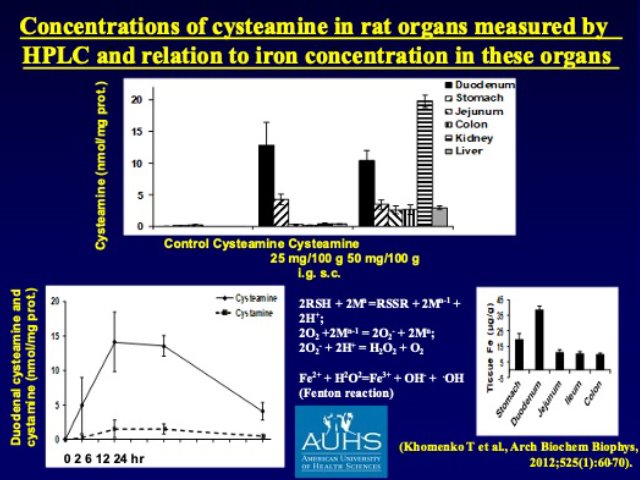
Yet, these studies did not explain why the lesions develop only in the duodenum, not in other organs, even after systemic (p.o. or s.c) administration of cysteamine. It was Dr. T. Khomenko, one of my creative coworkers who reminded us about the high concentration of iron (Fe) in the duodenal mucosa that provided the first rational explanation of the highly specific localization of cell & tissue injury in the duodenum. Namely, organ distribution studies with radioactive cysteamine showed very high concertation of cysteamine in the duodenum, where interactions with high levels of endogenous iron created a ‘perfect storm’ via the Fenton reaction when reactive the reactive oxygen-derived free radicals initiated a very rapidly developing cell & tissue injury that was partially prevented by antioxidants such superoxide dismutase. The most recent breakthrough for the explanation of cysteamine-induced DU is emerging from the experiments of Dr. J. Schloss who recognized the same molecular/structural specificity for certain enzymes that is identical with the in vivo DU potency of cysteamine vs. ethanolamine.
Mesenteric Fat: Recent data and perspectives for study.
Oksana Zayachkivska, MD, PhD, DSc
In modern times, the prevalence of metabolic disorders in the world has pandemic levels. Among them, obesity has similarly faced an upward trend, with the older population showing more susceptibility to obesity and related disorders than younger adults. One interpretation of the ability of adipose tissue to reprogram whole-body physiology is their mitochondria which integrate several processes, including oxidative phosphorylation, ATP synthesis, and free radical generation that could cause metabolic signals for obesity and vascular disorders. Mesenteric fat after 28-days hypercaloric diet could be source of systemic changes in cytoprotection. Our translational study has showed that using H2S signaling enrichment will help decrease mesenteric low-grade inflammation, inhibit of leucocyte-endothelial adhesion, mitochondrial dynamics in cell homeostasis, and keep redox balance. It could be implemented as a novel therapeutic strategy for decreased mesenteric visceral “cytotoxicity”.
The potential role of thiol dioxygenases in GI injury and carcinogenesis: New studies with cysteamine and its derivatives
John Schloss, Ph.D. Co-authors: Sandor Szabo, Jay Vadgama, Marguerita Lim-Wilby, Garrett Morris, Pranabananda Dutta, and Eduard Karapetyan
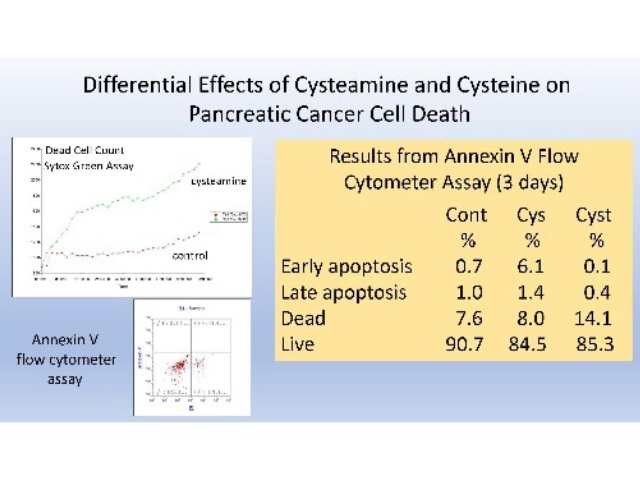
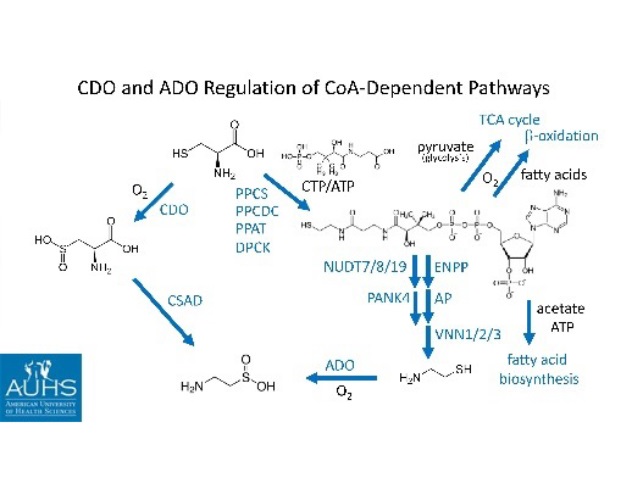
The molecular mechanism of cysteamine-induced duodenal ulcers and the effect of cysteamine on pancreatic cancer cells was addressed. Cysteamine was one of the first substances shown to produce perforating duodenal ulcers in a classic study by Selye and Szabo.1 Cysteamine has also been proposed as an adjunct therapy for pancreatic cancer based on its prevention of metastases in an animal model.2,3 It was shown that both effects are likely mediated by the enzyme cysteamine dioxygenase (ADO) based on (1) the differential effects of cysteamine and cysteine on ADO, (2) molecular modeling studies of the ADO enzyme, and (3) the inhibition of cell migration and cysteamine cytotoxicity against pancreatic cancer cells in culture. Cysteamine, but not cysteine, can reduce O2-oxidized ADO, producing cytotoxic superoxide in higher oxygen environments encountered by metastasizing cancer cells. This differential effect of cysteamine and cysteine can be readily explained based on molecular modeling studies conducted with mouse and human ADO crystal structures. Both cysteamine and cysteine inhibit the migration of pancreatic cancer cells in culture, but cysteamine and cysteine have different cytotoxic effects. Cysteamine increases the rate of cell death, but reduces the accumulation of apoptotic cells, while cysteine does not increase the population of dead cells, but does increase the accumulation of apoptotic cells relative to control cultures, based on Sytox Green and flow cytometry-Annexin V analyses. These results suggest that the role of iron in cysteamine mediated duodenal ulcers (Szabo abstract presented in the same meeting) is not due to free iron (classical ferroptosis), but to ADO-bound ferrous iron. ADO is likely inducible by cysteamine in both duodenal and pancreatic cancer cells. For pancreatic cancer cells this is consistent with the role ADO has in Coenzyme A degradation during the metabolic shift from aerobic metabolism to anaerobic glycolysis in transformed cells.
1Selye H, Szabó S (1973) Experimental Model for Production of Perforating Duodenal Ulcers by Cysteamine in the Rat, Nature 244(5416):458-9.
2Fujisawa T, Rubin B, Suzuki A, Patel PS, Gahl WA, Joshi BH, Puri RK (2012) Cysteamine suppresses invasion, metastasis and prolongs survival by inhibiting matrix metalloproteinases in a mouse model of human pancreatic cancer, PLoS One 7(4):e34437.
3EU/3/14/1252: Orphan designation for the treatment of pancreatic cancer.